Abstract
Background: For patients without HLA matched donor, Haploid stem cell transplantation with PTCY method has relatively higher risk of relapse and graft-versus-host disease (GVHD). Umbilical cord blood (UCB) is readily available and has helped expand the donor pool to almost all patients requiring a transplant. Meanwhile UCB transplant improves EFS in leukaemia with lower risk of relapse and GVHD. However, the clinical application of UCB is limited due to lower implantation rate because of its low dose of stem cells than other sources of hematopoietic stem cells (HSC).
Aims: This study is to explore the feasibility and efficacy of post-transplantation fludarabine and cyclophosphamide to select and promote the unrelated UCB to engraft in combined transplantation of haploid and UCB stem cells for the treatment of childhood and adolescent leukemia.
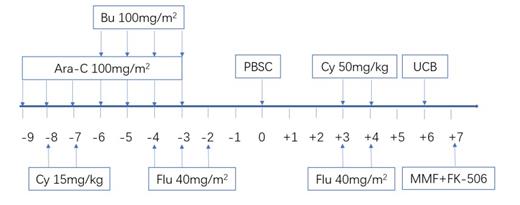
Methods: Total 40 children and adolescent patients with leukemia in Nanfang Hospital and Shenzhen Children's Hospital enrolled this study from Sept.2019 to Jun.2021. These patients were diagnosed as AML (20 HR, 5 IR, 2 relapsed AML), ALL (5 HR, 2 IR, 2 relapsed ALL), AL (1), JMML (2), MDS (1) and BPDCN (1) with a median age of 80 months (range 7 months -17 years). The biggest body weight reached to 77kg. The haploid stem cell was 5/10-9/10 mismatched and UCB was selected as 6/10-10/10 HLA matched or mismatched. The condition regimen was busulfan+fludarabine+CY+Ara-C. Haploid PBSC was infused in day0. All received PTCy 50 mg/kg and post-transplantation fludarabine 40mg/m 2 on days 3 and 4 along with tacrolimus or cyclosporine and mycophenolate mofetil for prophylaxis of acute GVHD. UCB was infused in day6. A median of haploid stem cells of 20.00×10 8/kg (13.00-32.10) of mononuclear cells was infused while a median of UCB cells of 4.32×10 7/kg (1.48-22.78) of total nucleated cells was infused. A median of CD34+ cells of haploid stem cells 13.00×10 6/kg (1.51-32.00) was infused while a median of CD34+ cells of UCB cells 1.74×10 5/kg (0.26-4.80) was infused. The survival rate, umbilical cord blood implantation rate, hematopoietic recovery and the rate of transplant-related complications were analyzed.
Results: At a median follow-up of 8 months (range 1-21 months), there were 2/40(5%)cases of transplant-related death. All surviving patients were leukemia free, with one-year overall survival rate 92.8±5.0%. Among these patients, 37/40(92.5%, 95%CI: 84.0%~100.0%) of patients achieved complete chimerism of unrelated UCB cells and 3/40(7.5%, 95%CI: -1.0%~16.0%) of patients achieved mixed chimerism of unrelated UCB cells and haploid cells. In these transpltantation, the CD34+ cell dose of UCB less than 1.0×10 5/kg accounted for 10/40 (25.0%), and less than 2.0×10 5/kg accounted for 23/40 (57.5%).Two patients had primary poor graft function. Neutrophil reconstitution was achieved in 39/40 patients with a median time of 29 days (range 17 - 44 days) without G-CSF after transplantation. Platelet recovery was achieved in 37/40 patients with a median time of 37 days (range 8-92 days). There was a significant linear relationship between platelet recovery time and the dose of total nucleated cells and CD34+ cells in UCB(r=-0.368, P=0.025; r=-0.355, P=0.031).The incidence of gradeⅠand gradeⅡGVHD was 32.5%(95%CI:17.3%-47.7%)and 42.5%(95%CI:26.5%-58.5%), respectively. There was no grade Ⅲ-Ⅳ aGVHD and only 1/40(2.5%) case of extensive chronic GVHD. The incidence of chronic limited GVHD was 22.5% (95%CI: 9.0%-36.0%). Twenty-four of 40(60.0%, 95%CI:44.1%-75.9%) patients experienced clinically significant CMV reactivations or infections. One of 40(2.5%, 95%CI: -2.6%-7.6%)patients experienced EB virus reactivation. Two of 40(5.0%, 95%CI: -2.1%-12.1%)patients experienced human herpesvirus 6 infection. Thirteen of 40(32.5%, 95%CI: 17.3%-47.7%)patients presented with hemorrhagic cystitis.
Conclusion: In our primary clinical study, post-transplantation fludarabine and cyclophosphamide could effectively select and promote the unrelated UCB to implant rather than haploid cells in combined transplantation of haploid and UCB stem cells even if the CD34+ cells of UCB less than 1.0×10 5/kg. Although acute GVHD was common but just milder degree and with lower incidence of EB virus reactivation. This new strategy has the potential to promote the wilder clinical use of unrelated UCB in the treatment of leukemia.
No relevant conflicts of interest to declare.